Funding Opportunity Description
Background
The Johns Hopkins University School of Medicine created the Center for POCTR for STDs (the Center) under an award from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) to coordinate development, clinical evaluation, and reduction to practice of new point-of-care (POC) devices. This Center is a collaboration between the Johns Hopkins University School of Medicine, the Johns Hopkins University Applied Physics Laboratory, the Infectious Diseases Institute at Makerere University College of Health Sciences in Uganda, and Cincinnati Children’s Hospital Medical Center. More information about the Center, its members, and the resources available to POCT developers can be found on the Center’s website.
The Center’s mission is to develop and test the accuracy, acceptability, and optimal implementation of point-of-care tests for sexually transmitted diseases in diverse care delivery contexts both in the United States and in resource-limited settings. Developed technologies are intended to bridge the gap between current biomedical sensors used in laboratory or research settings and those modified and optimized for use at the bedside, in the clinic, or in a home setting. The Center has conducted a number of needs assessments to understand the particular drivers for the list of requirements for POCT for STDs2-4.
The need to develop sensitive, specific, and more easily available POC technologies for diagnosing sexually transmitted diseases is critical. These infections are particularly problematic in women. Most STDs in women are asymptomatic and only detected through routine screening or when women present themselves for testing after being notified of infection in sexual partners or because they perceive themselves to be at risk. Five of the top ten reportable diseases to the Centers for Disease Control and Prevention (CDC) in the United States are STDs5. Chronic on going infections with these organisms may eventually lead to more serious conditions such as pelvic inflammatory disease and sterility.
Operational Environments and End User Preferences
POCT have been proposed for various settings. It is important that developers have a “use case” in mind when they are proposing a POCT. The optimal test characteristics for different operating environments vary.2,4,8 Table 3 shows possible operating environments where POCT would be beneficial and the characteristics associated with these settings.
Table 3: POCT Operational Environment and Characteristics Associated with Each
|
Operational Environment |
Average Patient Visit Time (Hours) |
Preferred Power Choices |
Storage of Assay Reagents |
|
Emergency Department (ED) |
4< |
120V (commercial power) |
Cold storage possible to -20o C |
|
Urgent Care Clinic or Community Clinic (UC) |
1> |
120V (commercial power) |
Highly variable: Cold storage possible to 4o C, -20o C or none |
|
Home Care (HT) |
0.25 (suggested) |
Non-commercial power such as batteries (rechargeable ) |
Reliable cold storage below 4o C may not be possible |
|
Low Resource Setting Clinic (LRS) |
2> Varies widely |
Batteries but not rechargeable (due to lack of consistent commercial power) |
No reliable cold storage |
It should be noted that the users (the people conducting the POC tests) may be significantly different in each of these operational environments and may include medical doctors, other trained health professionals, or lay people without medical backgrounds. In some cases, the patients would be expected to self-collect a sample and/or run the tests themselves6,7.
Research Objectives
While the types of POC technologies considered will include both novel detection technologies and novel enabling technologies, this solicitation is seeking primarily to provide “tactical” funding to develop or improve on novel detection technologies. Detection technologies are defined as technologies in which the device is able to identify and discriminate the infectious agent using a clinically relevant sample. Enabling technologies are defined as technologies which can be used with currently available diagnostic rapid tests to improve and simplify sample preparation or rapid development of new specific reagents (antibodies, aptamers, etc.) for use in existing detectors with potential to be transferred into a health care setting or home use.
“Tactical” funding is directed to a critical experiment(s) which, if successful, would 1) provide preliminary data, 2) enable first demonstration, 3) verify proof of principle/concept or 4) complete a seedling effort to enable organizations to seek additional funding for more robust technology development. These awards are narrow in scope, but should open the path to more robust and detailed development or integration of detection and/or enabling technology.
Infectious Targets
Preference in this solicitation is given to tests which definitively diagnose syphilis or discriminate syndromically related treatable STDs such as Chlamydia trachomatis (Ct), Trichomonas vaginalis (Tv), Neisseria gonorrhoeae (Ng), or Mycoplasma genitalium (Mg). These organisms are listed as examples only. Other organisms that cause STDs (with the exclusion of Zika virus) and are treatable infections will be considered. Assays which can detect active Syphilis infections are particularly of interest.
Detection can be achieved by:
- Detecting genetic or protein-based components of the organism
Or - Detecting combinations of general optical or electrical characteristics which can be determined to be unique to the organism
OR - Detecting surrogate markers as long as the clinical significance of the surrogate marker is well established
Comparative measures of sensitivity will vary by organism9. However, for Chlamydia, a successful technology would be expected to achieve an analytic detection of less than 1,000 elementary bodies/mL when presented in relevant diluents, which include phosphate buffered saline, DEPC treated water, or TRIS borate buffer. If required for the success of the detection technology, protocols for sample concentration must be included as part of the assay protocol.
Other acceptable measures of sensitivity would be technologies that achieve a sensitivity ≥ 90% when compared to the current clinical reference standard. For Chlamydia, the current accepted clinical reference standard is a vaginal or urine nucleic acid amplification test (NAAT), according to the CDC10. The developed test must show high specificity of the organism compared to expected confounding or commensal organisms. For the example of Chlamydia trachomatis, the technology would be expected to differentiate between C. trachomatis, C. pneumoniae and C. psittaci. Similarly, an assay for Neisseria gonorrhoeae should not detect non-pathogenic Neisseria, such as N. subflava, N. cinnerea, N. lactamica, etc. Another measure of specificity is a technology that achieves > 97% specificity compared to the current clinical reference standard. It is expected that the pre-clinical development will include testing of the diagnostic assay versus known dilutions of cultured organisms in order to determine the limit of detection (LOD).
Evaluation of detection technologies under consideration for award will include an assessment of the following technology performance metrics8,9:
- Achieves an analytic detection of low numbers of target STD. This value will vary according to the STD selected for detection. For example, Ct infections tend to present with low numbers of elementary bodies (EB<1000) shed during an infection while Ng infections tend to present with numbers significantly higher (>5000).
- Achieves a sensitivity ≥ 90% when compared to the current clinically accepted assay (also referred to as a “gold standard” assay).
- Demonstrates greater specificity to the target organism compared to expected confounding or commensal organisms.
- Demonstrates a detection time from sample collection to result in 30 minutes or less in a relevant operational setting (ED, UC, LRS, or HT).
- Demonstrates successful implementation by an inexperienced user, such as a non-laboratorian, with or without training. If intended to be CLIA waived, the test should not include more than 3 steps (exclusive of sample acquisition).
- Must be prepared for storage at room temperature (18-40oC) or 4oC for at least 6 months.
- Assay answer /readout must preferably not be subjective unless the end user is located in a resource-limited setting.
- Tests include all necessary controls for quality assurance and assay performance.
Other General Notes about the Research Objectives
- Proposals that describe component systems which have not been integrated or breadboard systems with preliminary results are eligible under this solicitation but applicants must describe specifically how they intend to reach the developmental level required to participate in testing of clinical samples in future years.
- Proposals using component-based systems where some of the components are already in use in food, medical, or environmental markets should emphasize which components are under development and which are novel developments.
- The development award can be used to modify an existing laboratory developed test for use in an urgent care clinic or home market.
- Batched assays which can be developed for single use assays are acceptable under this proposal.
- Size, weight, and storage conditions of assay reagents are not restricted for entry into clinical evaluation.
- All devices must work with standard power (120V) or batteries with the exception of LRS environments where standard non-rechargeable batteries are preferred.
Further Details about Successful Test Characteristics
- Multiplex detection technologies are desired which can discriminate Chlamydia from other important STDs including Neisseria spp., Trichomonas spp., Staphylococcus spp., Acinetobacter spp., and Candida albicans. Additional infections of interest to distinguish include Treponema pallidum, Haemophilus ducreyi, and Herpes Simplex virus (HSV).
- Protocols for use of the detection technologies should be written to accommodate users with at least an 8th grade reading level. Protocols should include all sample processing steps especially if sample concentration is necessary for successful detection. Protocols may be supplemented by up to one day of training for inexperienced users.
- Assay readout must be easy to read using a visual color change readout (subjective manual read of visual color change permitted in LRS only), digital, or graphic formats. Tests must include controls for interpretation of positive and negative values and a control for verification of assay performance. There are no requirements regarding the final size or packaging of the device at completion of funding.
Review Criteria
All reviewers and Center staff with access to proposals have completed conflict of interest and non-disclosure agreements ensuring confidentiality of the proposals.
Expressions of Interest
EOIs submitted by the deadline (Table 2) will be evaluated by Center staff for the following criteria:
- Has achieved a maturity level of “Proof of Concept” or above based on the GAITS criteria
- development of a prototype(s)
- supportive experimental results
- institutional IP disclosure
- Meets or exceeds ASSURED criteria
- The importance and number of STD(s) species detected
- Innovation /novelty of the approach
EOIs determined to meet the requirements outlined in this solicitation will be recommended for full proposals in order to elaborate on the proposed POCT solution.
Full Proposals
All full proposals will be evaluated by at least three independent scientific reviewers who are external to the Center. Full proposals will be evaluated based on the following criteria:
- Scientific and technical merit
- Potential to apply technology to common STDs using multiplex diagnostic testing
- The ability to fulfill the specific research objectives as stated above
- Significance and relevance to the mission of the Center
- Pre-analytical efficiency and rapid analytical speed of assay using laboratory strains
- Sensitivity and specificity compared to gold standard assay results
- Limit of detection (LOD)
- Storage conditions not lower than 4oC
- Appropriate quality assurance provision (e.g., assay and device controls, within performance/calibration controls for devices)
- Appropriately scoped to the amount of funding and period of performance requirements
- Competence and experience of the investigative team
- Bioengineering and research environment in which the work will be performed
- Suitability for use in near-patient applications in ED, UC, LRS, or HT
- Incorporation of testing principles or assay improvements that can achieve proof of feasibility or a higher maturity level within the next two years
- Plan for effective evaluation of the diagnostic method using archived clinical samples
General Award Information
A technology monitor from JHU APL will meet with successful awardees monthly to review progress and achievement of critical milestones and deliverables. As required by NIBIB, our Center will track the progress of awards using the GAITS platform.
The GAITS platform is based on the Healthcare Innovation Cycle (Figure 1) and structures work packages in deliverables and milestones.
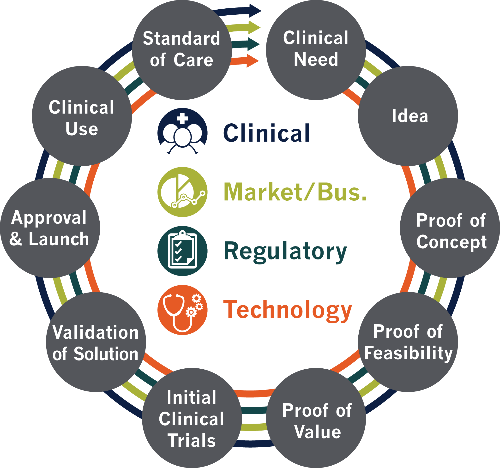
Figure 1: GAITS Healthcare Innovation Cycle
It is important to note that a single $50,000 USD award is not expected to address all of the maturity levels or all of the domains shown above especially since proposals should have achieved at least technological (technical readiness level) TRL 3 by the time of application (proof of concept).
A second six month award for technologies that are successful in their first period of performance will be considered if needed. The first award must be completed before a second award can be negotiated; however, it is permitted to apply for a second award in anticipation of completing the first award by the time the new proposal is reviewed.
The deadline for applications has passed
